Phases I and II of the NJMP sought to control and reduce the incidence of Johne’s disease. The objective for Phase III is unchanged and keeps the basic structure of the NJMP, which requires regular vet/farmer dialogue focusing on risk management and implementation of one of the six control strategies. This will continue to be backed up by annual certification.
To satisfy the requirements of the NJMP, there are three steps that should be completed on-farm with a BCVA-Accredited Johne’s Veterinary Advisor. These are:
- Know your Johne’s disease risks. While it is estimated that a third of dairy herds do not have Johne’s disease present on farm, a robust plan to keep the disease out – or manage the infection if present, needs to be worked up with a BAJVA. If your risks of Johne’s disease are high, it is important that your educe them by adopting an effective control programme and monitor carefully for infection in your herd.
- Know your Johne’s disease status. Testing will help determine your herd’s Johne’s disease status and the prevalence level in your herd. For Phase III, it has become a requirement for all herds to obtain an Average Test Value (ATV) for their herd to help assess the level of disease present and allow progress to be tracked over time. The minimum testing requirement to obtain an ATV is a 60 cow random screen.
- Create a written Johne’s disease management plan.
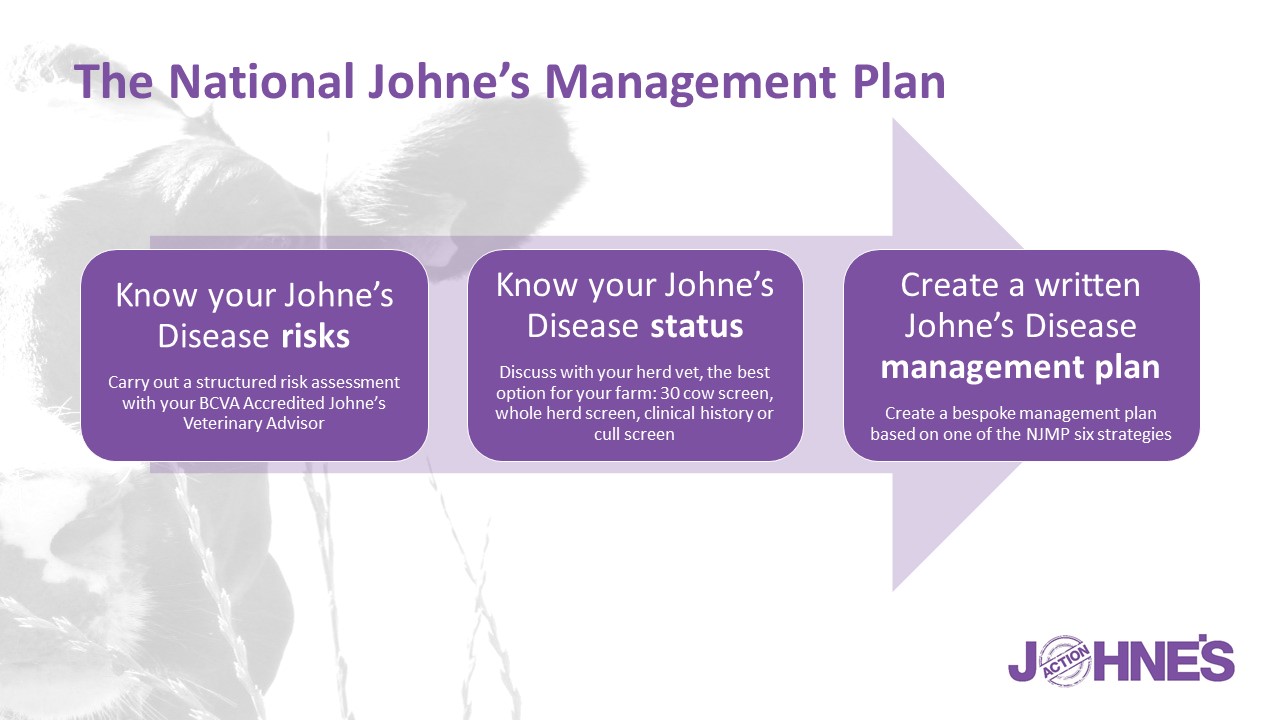
You should agree your Johne’s disease management plan with your BAJVA and review it at least once a year. You will still be required to complete an annual declaration. This annual declaration now includes space to record your ATV and to include three SMART (specific, measurable, achievable, realistic and time-bound) priorities to focus on over the year.
Only vets who have undergone the BCVA training programme, including all update modules, are permitted to sign declarations. Find your nearest BAJVA here.
Full details of the NJMP scheme can be found here: NJMP Phase III document.
